Adacel and Adacel Polio - notification about the identical Product Code
Notification about non-compliance with the DELEGATED REGULATION (EU) 2016 related to the medicinal products Adacel and Adacel Polio - UPDATE
In cooperation with the State institute for Drug Control, the marketing authorization holder, Sanofi Pasteur, has issued a notification about the presence of the identical PC (Product Code) on the outer packaging of 2 different presentations of the medicinal product Adacel.
Two presentations of the medicinal product Adacel that are different in the count of the needles enclosed (1 or 2 needles) contain the identical PC (Product Code) on the outer packaging in both human readable and encoded 2D format.
The identical PC is registered with the following batches:
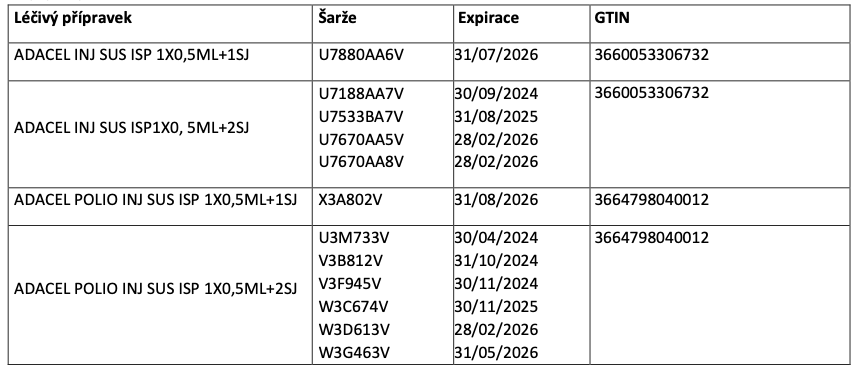
Further information and recommendations:
The event does not affect quality, safety, or effectivity of the medicinal product. The traceability of each pack is preserved, and no alerts are raised. The event is detected in case the end user stocks packs in their SW system by PC. The manufacturer has been prompted to perform investigation.
Further information including contact details can be found on the website of SÚKL.

 Registration and login to systems
Registration and login to systems












