5 years of successful verification of medicinal products
The Czech Medicines Verification Organization still ranks among the best within the EU.
On 9th February 2024 we celebrated the 5th anniversary of the launch of EMVS and CZMVS.
Medicines dispensed in the Czech brick-and-mortar pharmacies are deemed safe. This is achieved, among other things, thanks to the presence of the medicines verification system, which has been in place throughout the whole European Union since 2019. The Falsified Medicines Directive, 2011/62/EU, FMD has been effective as of 9.2.2019. All prescription medicines manufactured after this date must contain safety features enabling verification of authenticity, identification of each individual pack and ensure the pack has not been tampered with. As a result of the European regulation, the Czech Medicines Verification Organization (CZMVO) was also established, and after five years of the system's existence, it is one of the top three in the EU.
The likelihood of a falsified medicine being present in the Czech supply chain is negligible. However, within the EU, cases of medicines identified as forged/stolen are on the rise. Several signals have been obtained indicating existence of falsified packs of Ozempic (active ingredient Semaglutid), whose application led to severe undesirable effects. The verification of medicines hence protects safety of the European patients.
The Czech Medicines Verification System has been operating without problems for a long time. The system availability reached 99,94 % in 2023. The CZMVS was stable all year long and only very occasionally was there a temporary reduction in operation or a significant extension in response to requests from end users (pharmacies, warehouses). System functionality is critical for a smooth dispensation of drugs to patients. Currently, 34 different IT software providers supply information systems to pharmacies and wholesalers that are connected to CZMVS. In 2023, all IT companies were re-certified and upgraded to the latest API version.
During verification of medicines, alerts can be raised. If the verification fails, the system indicates a potential counterfeit. Alerts can be triggered also due to incorrect setup of the scanning device, missing or wrong data in the central repository (error on the part of the manufacturer or the On-Boarding-Partner), procedural error made following an inappropriate handling, etc. Before the concerned pack is declared a counterfeit suspect and reported to the authorities (in particular to the State Institute for Drug Control), these potential errors should be excluded (in the meanwhile, the pack is in quarantine). If the investigation turns out the alert is false positive, the pack can be dispensed to public. The authenticity is verified primarily in pharmacies and, in certain scenarios, also in wholesaler stocks.
Verification from the pan-European perspective
The European Medicines Verification Organization (EMVO) emitted a summary report in January 2024 evaluating the status of medicines verification at the pan-European and national level.
The monthly rate of alerts throughout EMVS is continuously declining and attacking the target rate of 0,05 %. For the last 2 years, the Czech Republic has been among the top 3 countries with the lowest count of alerts in relation to the total number of transactions and has been meeting the target in the long term. In 2023, the count of alerts in relation to the total number of transactions decreased in all countries and remained below 0,11 %. In comparison, in 2022 the ratio of alerts peaked at 0,34 % (week 1) and 0,21 % (week 22).
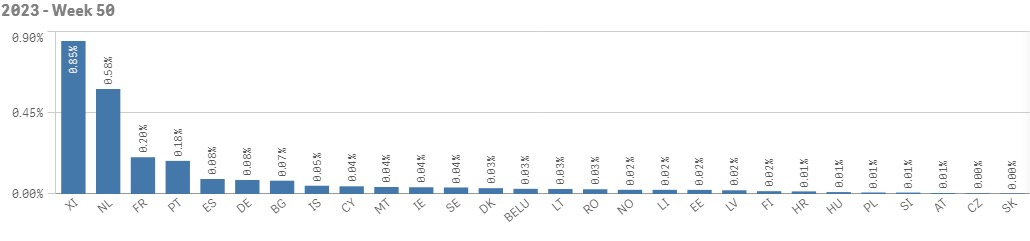
The highest total number of alerts, as per EMVO statistics, is reported by Spain followed by France, Germany and Netherlands. The highest count of alerts in relation to total number of scans is reported by Northern Ireland (see chart 1 and 2).
- The majority of alerts (all categories) was raised in France, Netherlands, Germany, Spain, Portugal and Romania. In Northern Ireland, the monthly share of alerts reached almost 0,9 %.
- 20 out of 28 European markets reached the target alert share of ≤ 0,05 %. The Czech Republic reports the lowest count of alerts in relation to the total number of transactions together with Slovakia.
Chart 1. The total count of alerts in each individual country in 2023
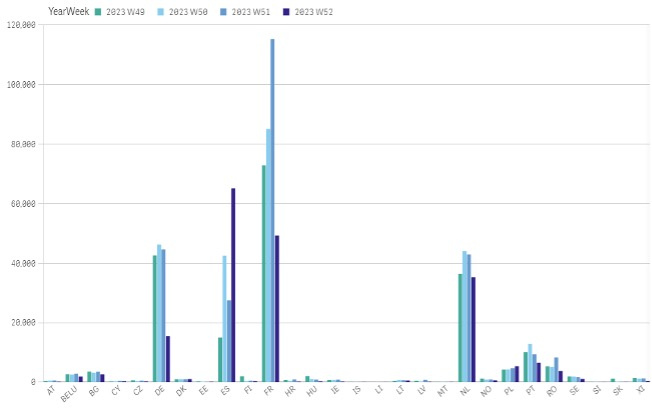
Chart 2. The relative count of alerts in relation to the total number of scans in week 50 of 2023
Czech Republic
The Czech Republic constantly appears in the group of the best countries with the share of alerts not exceeding 0,01 % together with Slovakia, Austria and Slovenia. The number of pharmacies and wholesalers connected to CZMVS is 100 %, comprised by 2964 pharmacies and 449 wholesaler stocks.
Alert management system
The Czech Republic was one of the first EU countries to offer alert management system (AMS) to end users facilitating alert investigation.
The State Institute for Drug Control has commented on the AMS:
Use of AMS in the process of alert resolution is supported by the State Institute for Drug Control. Hereunder is an extract from a statement posted in February 2023:
In compliance with the intention of the European Medicines Verification Organization (EMVO), the Czech Medicines Verification Organization (CZMVO) developed a national alert management system (AMS), which enables a fast resolution of alerts via either Application Programming Interface (API) or web interface. The AMS is being further developed in accordance with requirements of the European Alert Management System (EAMS).
AMS is a supportive system to the National Medicines Verification System (NMVS). The objective of the supportive system is to facilitate communication related to alert resolution, automate the whole alert investigation process, and share information. Finally, the system helps to reduce the count of „false positive” suspicions of counterfeit medicines.
The State Institute for Drug Control considers the use of AMS as a key tool to ensure control of medicines via safety features and calls upon marketing authorization holders, wholesalers and pharmacies to resolve and close their alerts via this system. The obligation to resolve all alerts is based on article 37/d of the COMMISSION DELEGATED REGULATION (EU) 2016/161. The CZMVO can only follow this obligation in cooperation with marketing authorization holders, wholesalers and pharmacies.
The AMS users are familiar with the system and know how to use it to resolve and close alerts. In 2023, over 97% of alerts were investigated and closed in cooperation between marketing authorization holders, pharmacies, wholesalers and CZMVS. The AMS currently provides access to 300 MAHs and over 840 organizations with 2268 locations. The number of connected entities is constantly growing. Within Europe, the Czech Republic is one of the countries with the highest involvement of users in alert management through AMS.
The latest information can be found at www.czmvo.cz

 Registration and login to systems
Registration and login to systems












